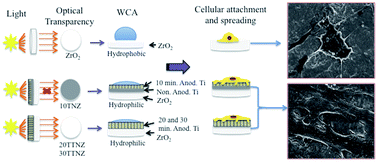
On Zirconia
Full contour zirconia (FCZir) now is being used routinely for everything from anterior single crowns and bridges to full-arch implant prostheses. Personally, I have found the esthetics of newer types of FCZir. An independent endorsement standing for Swarovski’s enduring commitment to the highest standards of excellence since 1895. The use of the special 'Zirconia from Swarovski' seal is a strong symbol of identification for the products of Swarovski Partners, enhancing their value and providing assurance that you are buying only the finest zirconia.
Alec Ganci, DDS, explains how it’s possible to bond zirconia as long as you effectively decontaminate and bond following the instructions of the cement manufacturer. He then presents a case study and review of Lava Esthetic Fluorescent Full-Contour Zirconia (3M).

Zirconia was introduced to dentistry in the early 2000s. For years, it had been used by the orthopedic industry for prosthetic joints due to its similarity to titanium, both in terms of strength and biocompatibility. When zirconia was first used in dentistry, it was regarded as an incredibly strong material used for bridge frameworks and single-unit copings. (1) However, it was too dull, white, and chalky to be considered for a posterior restoration—let alone anterior. (2) Luckily for the field of dentistry, things have changed.
Full contour zirconia (FCZir) now is being used routinely for everything from anterior single crowns and bridges to full-arch implant prostheses. Personally, I have found the esthetics of newer types of FCZir to be just as good as lithium disilicate, with the added benefits of higher strength and biocompatibility. The only fixed restorations for which I’m not using zirconia are veneers.
The problem with zirconia, however, is that when used in restorations, it has a penchant for falling off after insertion, sometimes after only a few weeks. To combat this issue, products had come out that were designed to treat the crown or the intaglio of the zirconia restoration. But unfortunately, the results of these products were unreliable. This is because our industry didn’t have a strong grasp on the mechanics and chemistry of bonding zirconia.
However, all this seems to be changing. One day, while using my intraoral scanner (True Definition Scanner, 3M), I noticed another option suddenly appear from the drop-down menu: Lava Esthetic Fluorescent Full-Contour Zirconia (3M). I was skeptical to try a new material (as I always am), but after speaking to my laboratory, we decided to give it a try.
The reasons Lava Esthetic interested us were as follows:
• Inherent, toothlike fluorescence
• High translucency
• Excellent shade match to Vita Classic shades, plus unique shading technology and built-in shade gradient
• Durable full-contour, with high-flexural strength (800 MPa) and fracture toughness (>3.5 MPa/m1/2)
• Strong, reliable bond
The next day, a good case opportunity presented itself. I had to prepare and scan No. 13 for a crown. I figured it was anteriorly positioned enough that I could test the esthetics, but it was posterior enough that the stakes weren’t too high. Nevertheless, you can see from Figures 1a–1c that we were thrilled with the results. Additional restoration examples can be seen in Figures 2 and 3.
Figures 1a–1c:
1a: Prepared No. 13, 1b: True Definition scan merged with clinical photo, 1c: No. 13 with Lava Esthetic full contour zirconia crown
The real question, however, was how well the FCZir bonded to the tooth. Nobody cares how lifelike a crown looks if it falls out a month after you’ve inserted it.
Figures 2 and 3: Additional restorations using Lava Esthetic
Bonding discoveries
For a few years now, I’ve bonded all of my crowns. In one office, I used Multilink Automix Next Generation (Ivoclar Vivadent), and in another I used RelyX Unicem 2 (3M). Both resin cements worked consistently as long as I followed the manufacturer’s instructions.
Before I go on, I should tell you that my dental philosophy is to take a few extra steps now to reduce treatment headaches later. This applies to lubricating provisional restorations with Vaseline, for example, and wrapping floss with a double knot in embrasures of pontics prior to temporary cementation to make excess cement removal a breeze.
To avoid dealing with the headache of an unhappy patient, I do what I can to ensure my crown doesn’t debond. But why not just use a luting cement and forget about trying to bond? Personally, I think luting cements are so white and opaque that they destroy any lifelike translucency we can achieve with glass and zirconia restorations. In other words, don’t worry about taking a stump shade if you’re using a luting cement because all of the natural shading of the prepared tooth will be blocked out by the luting cement.
Say you are using a resin cement to bond an indirect restoration. After trying in the crown and preparing to insert, you’re typically supposed to do the following:
• Clean the tooth (i.e., use pumice).
• Decontaminate the intaglio of the restoration.
• Prepare the intaglio with some type of adhesive or priming agent to link the cement to the crown. (Some brands of cement, like Unicem, don’t require a separate primer because it’s already built in.)
• Cement and tack cure, and then clean up the excess.
I began to realize, however, that the step I wasn’t paying much attention to was one of the most important ones: decontaminating the intaglio of the restoration, which is the true source of the zirconia debonding issue.
Bear with me as we discuss the chemistry involved: The zirconia we use in dentistry is zirconium oxide, and the oxide part is where all the bonding happens. It is the functional phosphate groups that bond to the zirconia. But wait, what else contains tons of phosphate? That’s right, saliva. Therefore, once a crown is tried in the patient’s mouth, all of the potential bonding sites on the zirconia crown are now irreversibly occupied by phosphate groups. (3,4) Once this happens, we need to remove these phosphate groups to make the oxide sites ready to bond again.
This is easier said than done. Wiping with alcohol doesn’t do anything. Sandblasting can do it—however, most manufacturers recommend you leave the sandblasting to your laboratory. Some claim that excess microetching with aluminum oxide can increase future fracture risk. However, microetching is an important step because it’s theorized to increase bond strength by increasing surface energy of the intaglio surface. (5,6) Porcelain etch (hydrofluoric acid), although helpful for glass restorations like lithium disilicate, doesn’t do anything clinically relevant to zirconia. Oh, and phosphoric acid etch? Big no-no. Phosphoric etch just makes the problem worse by flooding whatever oxide groups aren’t already occupied by phosphate with, you guessed it, phosphate groups.

How do we remove the phosphate from the oxide sites we need to bond to? There are two reliable methods: Ivoclean (Ivoclar Vivadent) and Sodium hypochlorite (NaOCl).
Ivoclean is superconcentrated zirconia oxide in a liquid suspension (so make sure to shake well before using). It acts like a magnet to remove all the phosphate from the crown by creating a concentration gradient. Rinse off after 20 seconds, and now you’ve got a zirconia crown with all of its oxide groups ready to accept a bond from your cement. (7)
Another recently discovered method is to just wipe the intaglio of the crown with gauze that is soaked in 5% sodium hypochlorite. The theory is that NaOCl breaks down the phosphate groups so they can no longer bond, and it can be removed by rinsing with water followed by air drying.8 Bleach, then bond—voilà!
In conclusion, it’s possible to bond zirconia as long as you (1) effectively decontaminate the tooth and the crown and (2) bond following the instructions of the cement manufacturer.
Barium On Zirconia
Author’s note: The author would like to thank Mark Hartslief, BSc, RDT, and the New York Center for Digital Restorative Solutions (NYCDRS) laboratory and knowledge center for fabricating the pictured restorations and their contribution to this case study. NYCDRS can be contacted at (646) 757-5840 or kim@nycdrs.org. Visit them at nycdrs.org.
Zirconia On Alumina
References
1. Seghi RR, Denry IL, Rosensteil SF. Relative fracture toughness and hardness of new dental ceramics. J Prosthet Dent. 1995;74(2):145-150.
2. Christel P, Meunier A, et al. Mechanical properties and short term in-vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J Biomed Mater Res. 1989;23(1):45-61.
3. Yang B, Lange-Jansen HC, Scharnberg M, et al. Influence of saliva contamination on zirconia ceramic bonding. Dent Mater. 2008;24(4):508-513.
4. Kweon HK, Hakansson K. Selective zirconium dioxide-based enrichment of phosphorylated peptides for mass spectrometric analysis. Anal Chem. 2006;78(6):1743-1749.

5. Blatz MB, Alvarez M, Sawyer K, Brindis M. How to bond to zirconia: the APC concept. Compend Contin Educ Dent. 2016;37(9):611-617.
6. Kern M. Bonding to oxide ceramics—laboratory testing versus clinical outcome. Dent Mater. 2015;31(1):8-14.
7. Wolfart M, Lehmann F, Wolfart S, Kern M. Durability of the resin bond strength to zirconia ceramic after using different surface conditioning methods. Dent Mater. 2007;23(1):45-50.
8. Rosentritt M, Behr M, Hahnel S, Preis V. Surface treatment on shear bond strength of high translucent zirconia. Poster presented at: International Association of Dental Research General Session (IADR); March 22-25, 2017; San Francisco, USA.
Alec J. Ganci, DDS, FICOI, attended Stony Brook School of Dental Medicine, completing an oral surgery externship in Madagascar. He continued his training as a resident at North Shore University Hospital, serving as chief resident, and completed a two-year fellowship in advanced prosthodontics and implant dentistry. He is an industry consultant and lectures extensively on various topics, including implants, dental materials, digital dentistry workflows, and esthetics. He can be reached at alec.ganci@gmail.com.
Worth knowing:
Cubic Zirconia Crown Dental
Properties of Zirconium Oxide (ZrO2)
- High thermal expansion (α=11 x 10-6/K, similar to some types of steel)
- Excellent thermal insulation/low thermal conductivity (2.5 to 3 W/mK)
- Very high resistance to crack propagation, high fracture toughness (6.5 to 8 MPam1/2)
- Ability to conduct oxygen ions (used for the measurement of oxygen partial pressures in lambda probes)
Another outstanding property combination is the very low thermal conductivity and high strength. In addition, some types of zirconium oxide ceramics can conduct oxygen ions. Components made from this material are significantly more expensive than components made of alumina ceramics. Zirconium oxide ceramics are used, among other applications, as tools for wire forming, as auxiliaries in welding processes, as materials for crowns and bridges in the dental industry, as insulating rings in thermal processes, and as oxygen measurement cells in lambda probes.